Join Us In The Fight Against The Coronavirus! Vir Biotechnology is conducting a research study for COVID-19 patients who have recovered from this infection. Consider donating your blood to help researchers better understand this virus. Email at virus@vir.bio or visit https://www.vir.bio/virus/
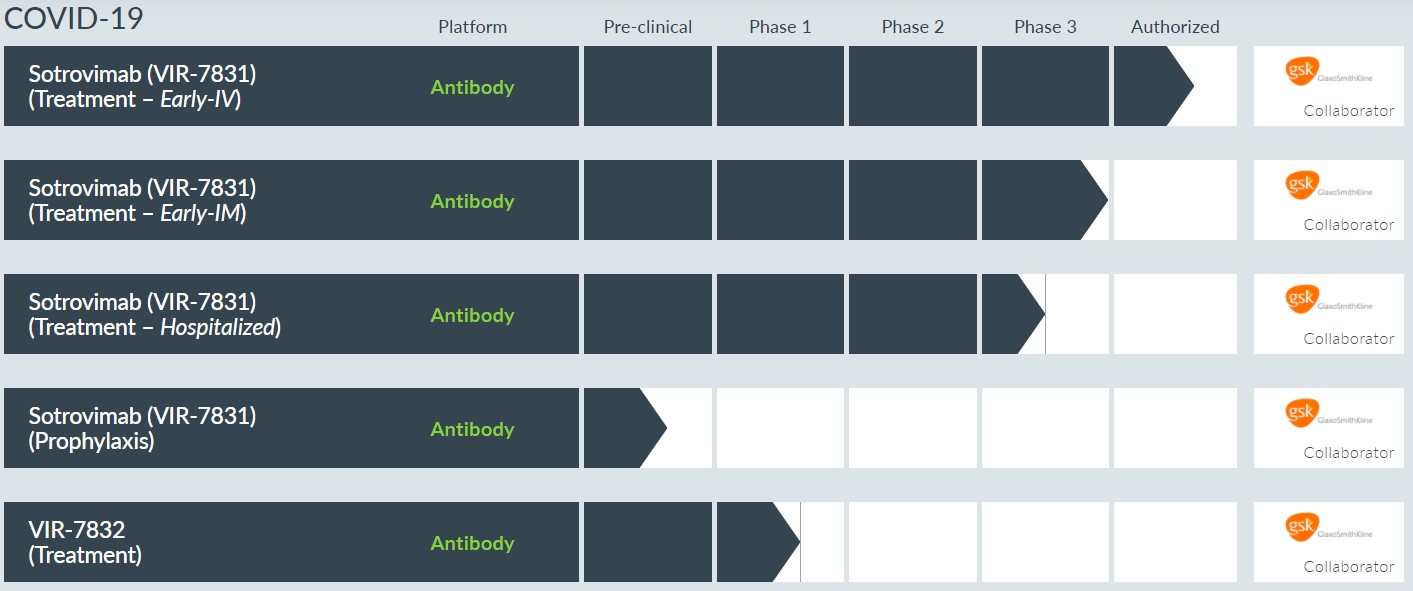
GSK and Vir Submit Emergency Use Authorization Application to FDA for Intramuscular Administration of Sotrovimab for the Early Treatment of COVID-19 - GlaxoSmithKline plc and Vir Biotechnology, Inc. announced the submission of an application to the FDA requesting an amendment to the Emergency Use Authorization (EUA) for sotrovimab, an investigational monoclonal antibody for the early treatment of COVID-19, to include intramuscular (IM) administration. The EUA for sotrovimab was granted by the FDA in May 2021 as an investigational single-dose intravenous (IV) (500 mg) infusion SARS-CoV-2 monoclonal antibody for the early treatment of COVID-19, and the companies are requesting an expansion to the EUA to also include IM administration (500 mg).
GSK and Vir Biotechnology Announce United States Government Agreement to Purchase Additional Supply of Sotrovimab, Authorized for the Early Treatment of COVID-19 - The US government will purchase an additional 600,000 doses of sotrovimab, an investigational monoclonal antibody for the early treatment of COVID-19, enabling further nationwide access to sotrovimab for patients. The additional 600,000 doses will be delivered throughout the first quarter of 2022. This agreement is an amendment to earlier commitments announced with the US government in November 2021. Preclinical data generated through both pseudo-virus and live virus testing demonstrate sotrovimab retains activity against all tested SARS-CoV-2 variants of concern including Delta and Omicron.
Vir Biotechnology Announces New Research Describing the Structural Basis of SARS-CoV-2 Omicron Immune Evasion and Receptor Engagement - New preclinical research published to the preprint server bioRxiv, describing the structural basis and magnitude by which the new SARS-CoV-2 Omicron variant (B.1.1.529) evades antibody mediated immunity, as well as its enhanced ability to bind to the human ACE-2 receptor. Data define the specific Omicron mutations and their detrimental impact on the binding of the majority of tested monoclonal antibody (mAbs) therapies that target the receptor binding motif of the spike protein, a region that is more prone to mutate. Further, these data add to the growing body of evidence from recent pseudo- and live virus neutralization findings demonstrating that sotrovimab retains activity against the Omicron variant, as well as all tested variants of concern. This study was conducted in close collaboration with David Veesler, Ph.D., Associate Professor of Biochemistry, University of Washington & Investigator, Howard Hughes Medical Institute, and members of his laboratory. Sotrovimab is an investigational SARS-CoV-2 neutralizing monoclonal antibody developed in partnership with GlaxoSmithKline for the early treatment of COVID-19.
Vir and Alnylam Expand Collaboration to Advance Investigational RNAi Therapeutics Targeting Host Factors for the Treatment of COVID-19 - This expansion includes up to three additional targets focused on host factors for SARS-CoV-2, including ACE2 and TMPRSS2, both of which are considered critical for viral entry, with the potential for an additional host target to emerge from Vir’s functional genomics work.
GSK and Vir Biotechnology enter collaboration to find coronavirus solutions - Promising antibody candidates for SARS-CoV-2 to be accelerated into phase 2 clinical trials within the next three to five months
