Single-Patient Expanded Access: An Infographic
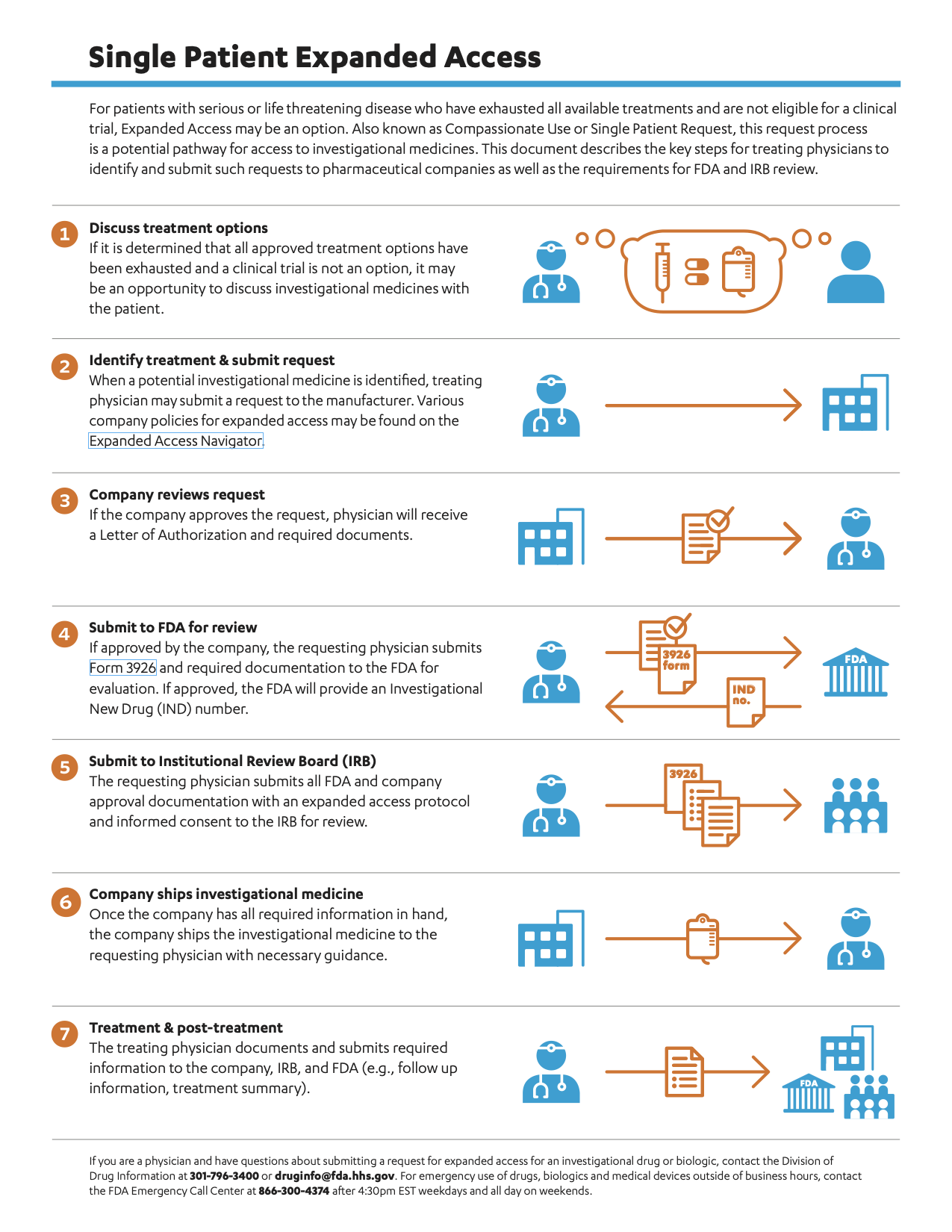
For patients with serious or life threatening disease who have exhausted all available treatments and are not eligible for a clinical trial, Expanded Access may be an option. Also known as Compassionate Use or Single Patient Request, this request process is a potential pathway for access to investigational medicines. This document describes the key steps for treating physicians to identify and submit such requests to pharmaceutical companies as well as the requirements for FDA and IRB review.